Application notes
Investigating powder caking in a short time with the GranuPack High Temperature
Unravel the mystery of powder caking in industries like food and pharmaceuticals. Prolonged storage triggers aggregation, unveiling a tale of quality challenges and intrigue.
Tapped density at high temperature: the new technique for investigating powder caking in a short time, using the:

Introduction
Generalities
Caking is generally a problem in industries handling powder material. Food and pharmaceutical industries are the principal fields impacted by this issue. Indeed the products are in powder form, packed in bags, and stored sometime during a long period before use or sale. During this time, the powder undergoes different temperatures, humidities, and loads, activating the caking process. It results in variability in the quality of the product, depending on the duration and the conditions that were undergone during the storage. The powder is aggregated and sometimes unusable.
Powder caking is due to phenomena that occur at the grain scale. The principal reason for caking is the formation of solid bridges between grains, leading to an irreversible change in powder flowability. Different mechanisms can be in cause:
- Dissolution and recrystallization of chemical compound during a cycle of moisture adsorption
and drying. This can happen for instance with salt or sugar dissolved in water. - Sintering of grains at temperatures close to the melting temperature.
- Plastic deformation of grain, at the contact point, increasing the contact area and thus the
adhesive forces (Van der Waals). - Chemical reaction.
- Biological processes like fungal growth.
- Solid bridge formation of amorphous material.
The process that will be discussed here is the caking due to the amorphous nature of grains. Indeed, food and pharmaceutical powders are mainly made of sugar polymers and amorphous materials such as lactose. The particularity of these materials is that they have a glass transition at a specific temperature Tg, meaning that under or beyond Tg, the material can be seen as a ‘’glassy solid’’ or in a ‘’rubbery state’’. In the rubbery state (T>Tg), the molecule mobility is large enough to observe deformation of the material at a laboratory timescale. In the glassy state (T<Tg), the material is commonly seen as a solid at the laboratory timescale. However, considering longer times, the low mobility of molecules at T<Tg is still sufficient to observe a plastic or ‘’liquid’’ deformation of the material. This means that the more the temperature is close or beyond Tg, the faster the material can be deformed due to molecule motion and lead to plastic deformation. Therefore, it is easier for an amorphous material to create solid bridges when the temperature is high. On the contrary, at low temperatures, below Tg, the formation of solid bridges is still possible but the timescale for the deformation is larger, far from a laboratory timecale.
Scope of the study
In this work, we investigated the effect of temperature on the caking process. We address the problem
of predicting the caking. More specifically, at which timescale it can start to appear. Indeed, many powder producers store their powder in sheds after packaging in bags. There, the powder can stay for weeks, under various humidities, temperatures, and pressures before being sent to customers. Therefore, the variability between different batches can be high depending on the different durations of the storage step. A big improvement would be to predict the typical time of caking to define a limit time of storage.
For such prediction, tests are performed on the staked bag or at the lab scale. Generally, these tests consist of conditioning the powder at a specific humidity and pressure for a long period of time (several days or weeks). These tests are challenging since they are conducted over a long period of time. Furthermore, the link between temperature and the glass transition of amorphous materials is generally poorly considered while performing tests at higher temperatures than room temperature allows to accelerate such investigation. Indeed, the caking takes place as fast as the temperature is close or higher than Tg. Therefore, increasing the temperature compared to the room temperature allows for accelerating caking test in a reasonable time, taking a few minutes to a few hours.
For each temperature, the typical caking time (the minimal time at which the powder starts to be caked) can be measured by determining the time (called here heating time) needed to reach the caked state. Taking into account the advantage of fast measurements at high temperatures, several points (temperature ; heating time) can be measured in a short time. From these points, the evolution of the typical caking time as a function of temperature can be extrapolated with a polynom as illustrated in Figure 1. Therefore the typical caking time at room temperature can be found by polynomial extrapolation from few data performed at high temperatures in a short time compared to experiments done at room temperature.
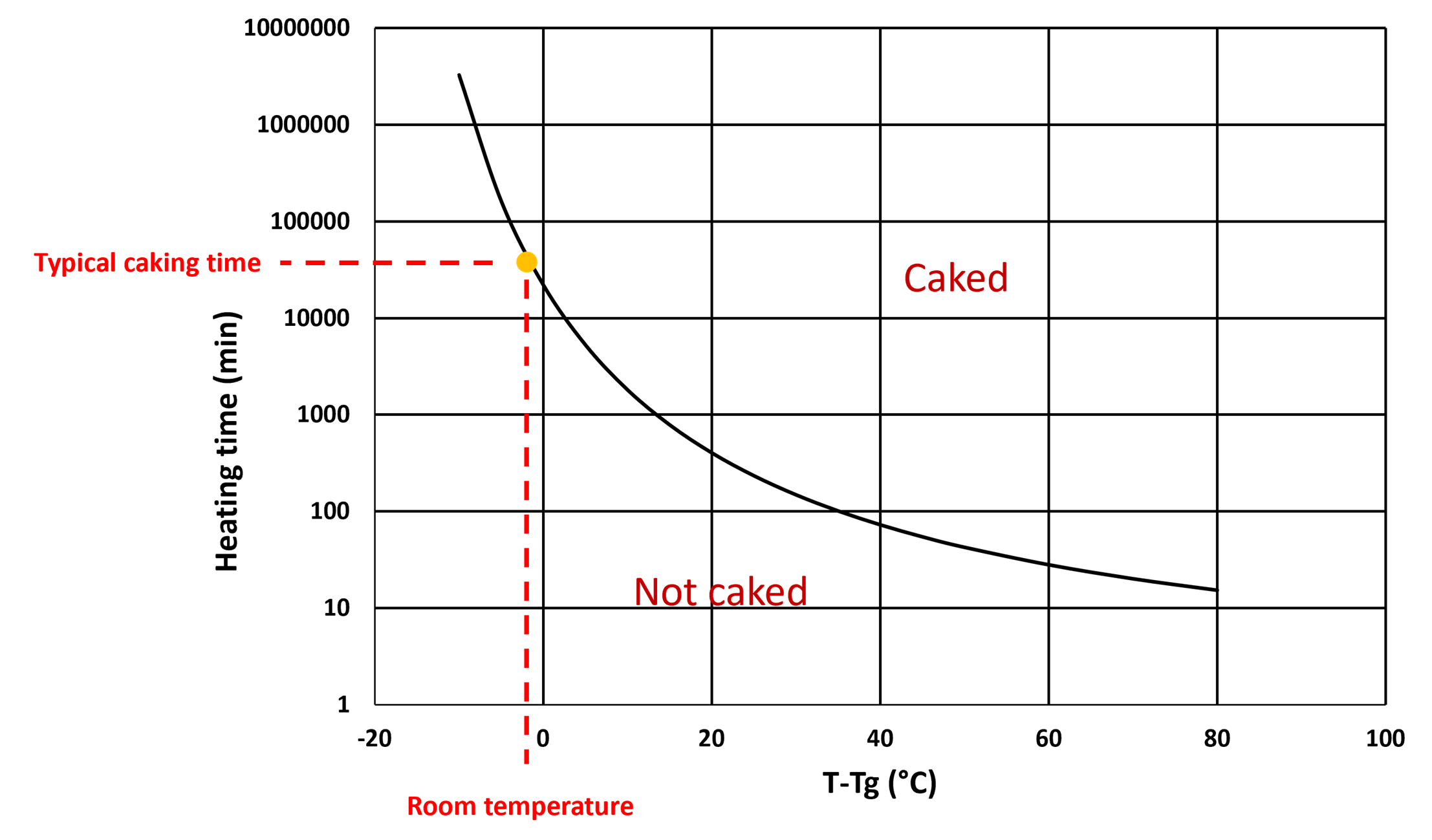
Figure 1: Scheme of the heating time for caking as a function of temperature. The black curve represents the evolution of the typical caking time extrapolated from data taken at high temperature.
LEARN MORE ABOUT THE GRANUPACK
Powders characteristics
For this study, lactose powder was employed as a model material. Indeed this powder is largely used in the food and pharmaceutical industries which are two big fields concerned by this problem. Its glass transition is around 101°C. The Pharmatose 450M® grade (from DFE pharma) was used. This grade was selected for its large size distribution (see Figure 2) that favors the caking.
Figure 2: SEM image of Pharmatose 450M®
Packing analysis
Experimental protocol
The evolution of caking with time and temperature was investigated with the GranuPack High Temperature (see Figure 3). This instrument performs tapped density measurement at a selected temperature from room to 200°C. It works as follows: the powder is poured in a metallic tube with an automated initialization protocol and a hollow cylinder is placed at the top of the powder to keep the interface flat. This tube performs a succession of free falls, called tap, to densify the powder. After each tap, the height of the granular pile is measured by a laser sensor. From this height h and the known tube diameter and mass of powder, the bulk density is computed. The packing amplitude, characterizing the amplitude of densification of the powder is defined as Δρ = ρ(n) - ρ(0), where ρ(0) is the initial bulk density and ρ(n) the tapped density after n taps. Here, n was fixed at 500. During the experiment, the temperature is controlled by the tube which is surrounded by an electrical heating jacket.
For this study, the following protocol was applied: after the initialization protocol, the powder was densified with 50 preliminary taps at room temperature. This step aimed to increase the density and thus the number of contacts between particles to favor caking before the measurement. Afterward, the powder was heated during a selected time, called ‘’heating time’’, at a target temperature. Once the powder was heated at the right temperature during the defined heating time, the densification started and the temperature was kept constant at the selected temperature. Different heating times were tested for two temperatures 110°C and 120°C. For each experiment with the GranuPack, 500 taps were applied to the sample with a tap frequency of 1Hz and 1 mm of free-fall. Measurements were repeated three times for evaluating reproducibility and the average value and standard deviation were considered. The different metrics considered are computed from the bulk density after the 50 preliminary taps.
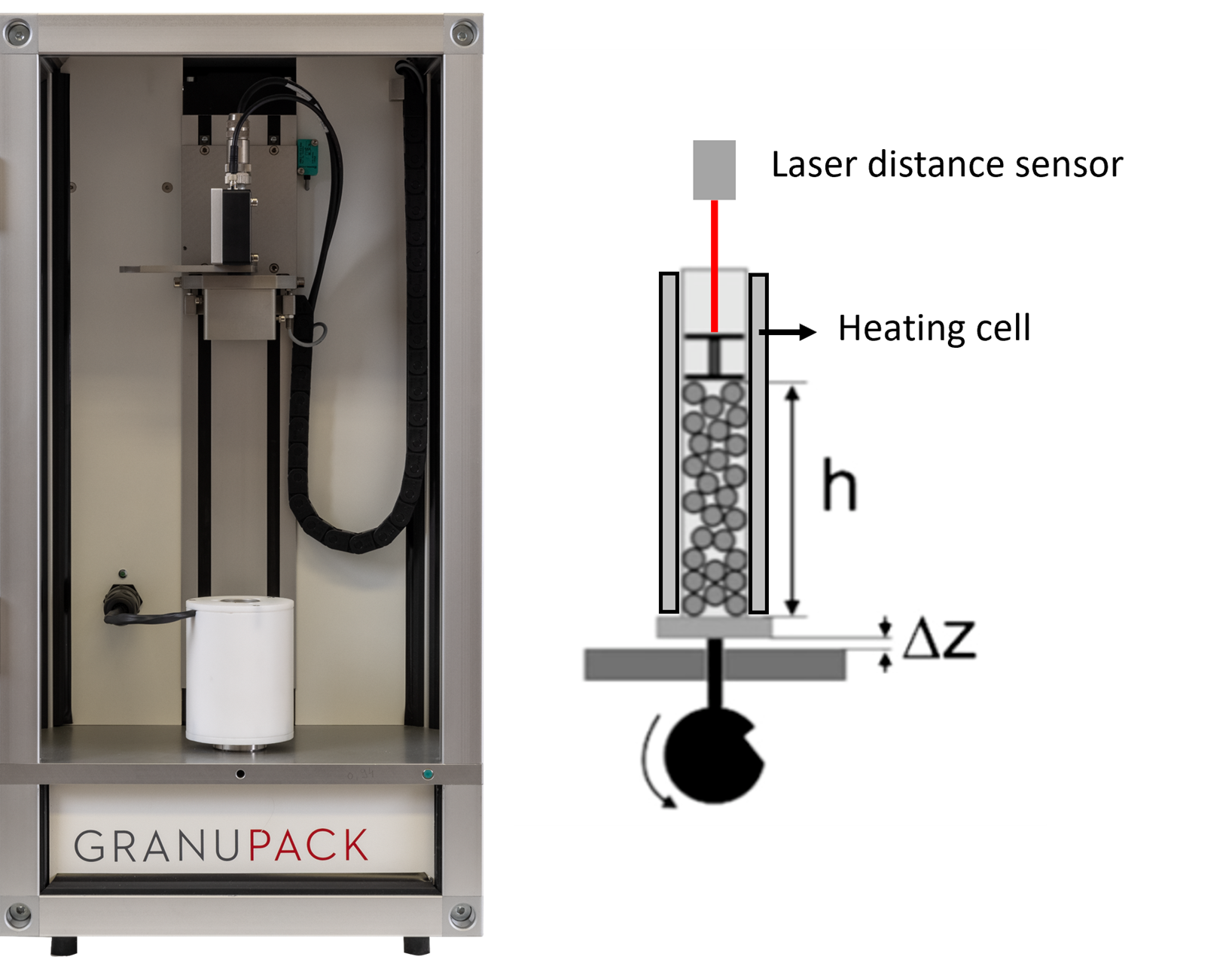
Figure 3: (left) Picture of the GranuPack High Temperature. (right) Sketch of the GranuPack High Temperature.
LEARN MORE ABOUT THE GRANUPACK
Experimental results
The amplitude of densification is highly influenced by the heating time. Indeed, a significant decrease in the final density ρ(500) with the heating time is observed for the Pharmatose 450M® powder heated at 110°C as illustrated in Figure 4. When the temperature is close enough to the glass transition, the typical time for particles to create solid bridges due to mobility of the amorphous lactose is decreased to a time closer to the experiment timescale. Therefore, the longer the powder is heated, the more solid bridges have been created. These bridges limit the grain reorganization and help the granular pile to sustain a loose packing, resulting in a reduction in the final density with the heating time. On the contrary, the initial packing remains unchanged since the initial granular pile is generated at room temperature. As a consequence, the packing amplitude decreases with the heating time and thus represents an adequate metric to characterize the evolution of caking with the heating time.
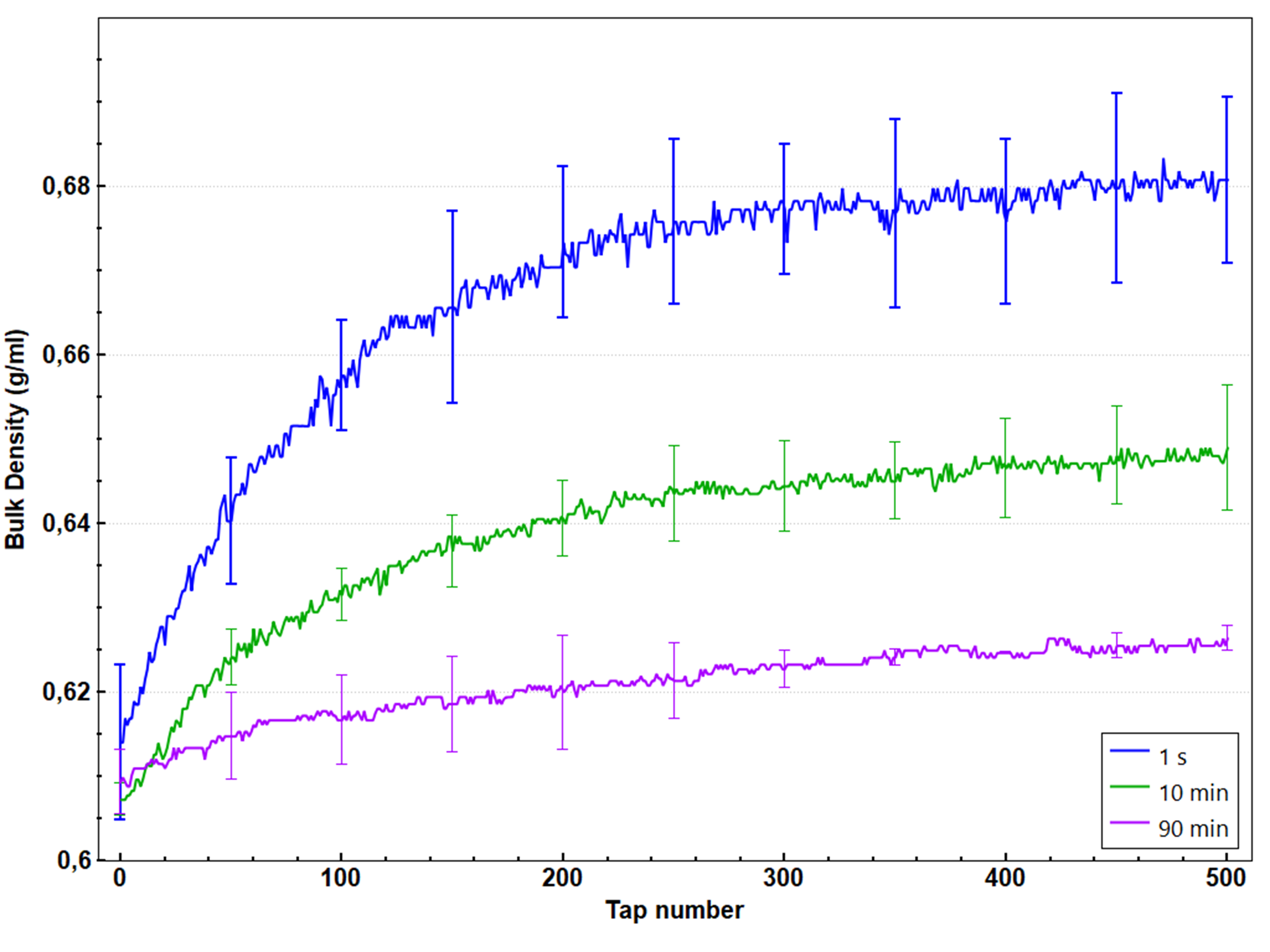
Figure 4: Bulk density variations versus tap number for different heating times. The measurement is done at 110°C.
Theoretically, a non-caked powder exhibits a significant and measurable increase in density. The more the powder becomes caked and creates solid bridges between particles the more Δρ decreases. Therefore, a caked powder should give Δρ=0 g/ml, meaning that it does not densify. However, this asymptotical value is not reached experimentally and a value larger but close to 0 g/ml is obtained.
Figure 5 presents the evolution of the packing amplitude with the heating time for two temperatures: 110°C and 120°C. For both temperatures, Δρ decreases as the heating time increases, indicating the intensification of the caking of the powder with the heating time. In both cases, the packing amplitude starts at ∆ρ≈0.60 g/ml and decreases until ∆ρ≈0.13 g/ml, corresponding to the caked state of the Pharmatose 450M®. The decrease in Δρ saturates at this latest value. A plateau can be observed as highlighted by the black horizontal line at about ∆ρ≈0.13 g/ml. The difference between 110°C and 120°C is the time at which this plateau is reached (corresponding to the caked state). Indeed, at 110°C, the saturation is reached after a typical time of τ≈1800 s while it is reached after typically τ≈900 s at 120°C, i.e. two times faster. Therefore, these typical times correspond to the typical caking time at respectively 110°C and 120°C.
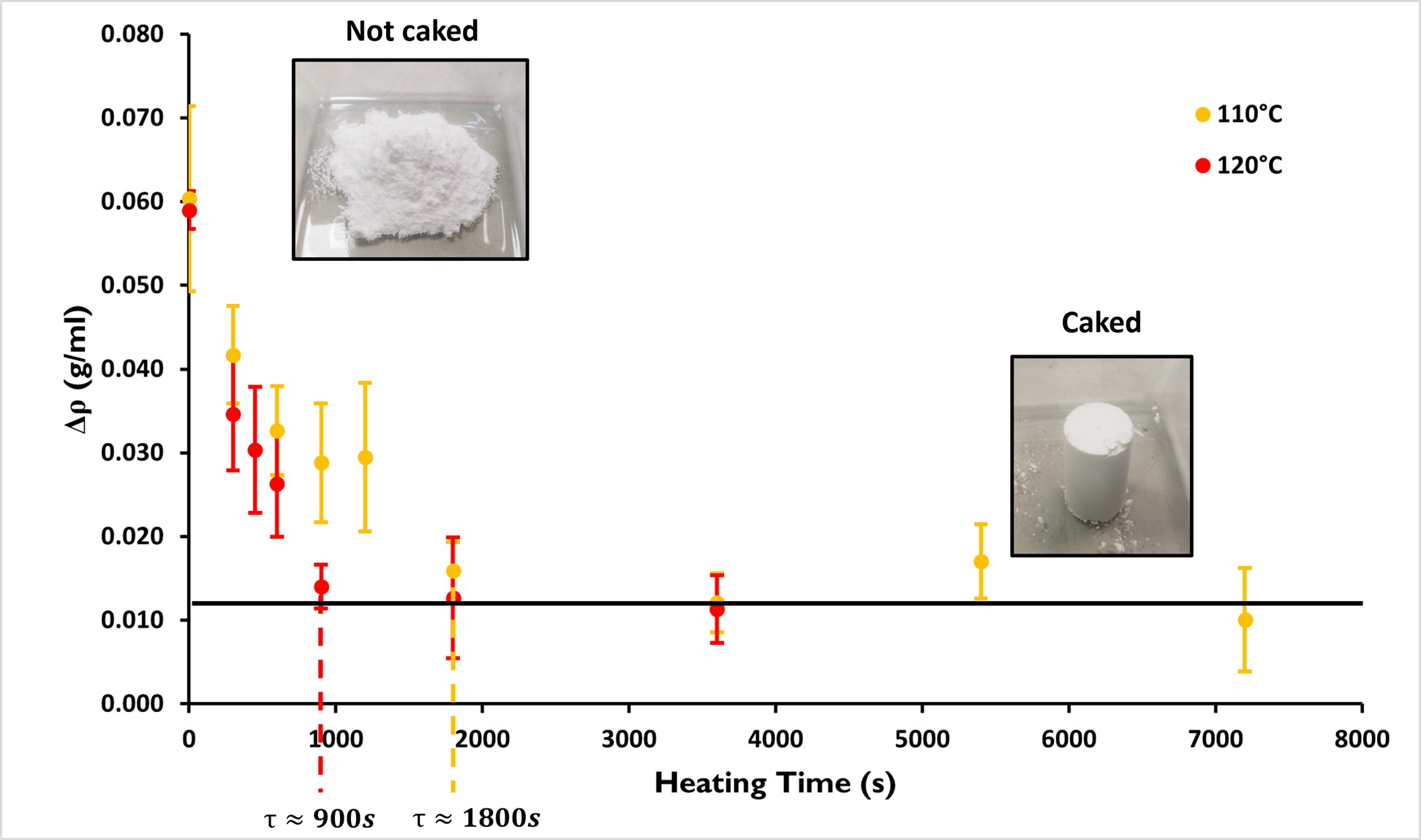
Figure 5: Evolution of the packing amplitude Δρ as a function of the heating time for Pharmatose 450M® at 110°C and 120°C.
This result is consistent with the caking due to the glass transition of lactose. The larger the temperature, the faster the formation of solid bridges. Compared to conditioning done at room temperature, taking several weeks or months, increasing the temperature allows the heating time to be reduced on a laboratory timescale, from minutes to hours. Therefore, the typical time for reaching the saturation value of Δρ, corresponding to the typical caking time, can be found for different temperatures in a reasonable time. By extrapolation, the typical caking time at room temperature could be found.
A new approach for predicting the typical caking time is possible by measuring the densification of powder at high temperatures instead of directly investigating the caking at room conditions during long-time experiments. This new approach suggests measuring the typical caking time for different temperatures, close to Tg. With several data, the typical caking time at room temperature could be found by extrapolation as illustrated in Figure 1.
Conclusions
Powder caking is a big issue to solve in the food and pharmaceutical industries. During storage, powder caking commonly takes place and decreases the quality of the powder. However, this caking takes time to appear. Therefore it is advisable to be able to estimate the typical caking time to avoid storing a powder longer than this limit time. A common approach consists of directly testing the powder in identical conditions as the storage to evaluate this time. Such investigations are complex and time-consuming as the timescale can vary from a few weeks to months.
In this work, we investigated the evolution of the typical caking time with temperature. Pharmatose 450M® powder was used. The packing amplitude was found to be a good metric to define this typical caking time. At a fixed temperature, the packing amplitude was observed to decrease with the heating time. This decrease saturates at a low value, close to 0 representing the theoretical Δρ at which the powder is completely caked. Therefore, for each temperature, the typical caking time can be defined as the heating time required to reach the saturation of the packing amplitude. Two temperatures (110°C and 120°C) were tested and a decrease in the typical caking time with temperature was observed in agreement with the glass transition theory.
Based on tapped density measurement at high temperature performed with the GranuPack High Temperature, typical caking times can be measured at different temperatures, in a reasonable time, from minutes to hours. Then the typical caking time at room temperature, which can take several weeks, could be extrapolated from a few data performed at high temperatures in a few hours. This new approach would provide a convenient way to estimate the typical caking time, avoiding storage longer than this limit time.
Acknowledgments
The authors thank Thomas Gemmine for its help in data acquisition.
