Chemicals
Influence of Humidity On Powders Surface Properties
Granular materials and fine powders are widely used in industrial applications. To control and to optimize processing methods, these materials have to be precisely characterized...
Introduction
Theoretical Framework
Granular materials and fine powders are widely used in industrial applications. To control and to optimize processing methods, these materials have to be precisely characterized. The characterization methods are related either to the properties of the grains (granulometry, morphology, chemical composition, …) and to the behaviour of the bulk powder (flowability, density, blend stability, electrostatic properties, …).
However, concerning the physical behaviour of bulk powder, most of the techniques used in R&D or quality control laboratories are based on old measurement techniques. During the last decade, we have updated these techniques to meet the present requirements of R&D laboratories and production departments. In particular, the measurement processes have been automatized and rigorous initialization methods have been developed to obtain reproducible and interpretable results.
Moreover, the use of image analysis techniques improves the measurements precision.
A range of measurement methods has been developed to cover all the needs of industries processing powders and granular materials.
However, in this application note, we will be focused on the GranuCharge instrument.
GranuCharge
Electrostatic charges are created inside a powder during a flow. This apparition of electric charges is due to the triboelectric effect, which is a charge exchange at the contact between two solids. During the flow of a powder inside a device (mixer, silo, conveyor, …), the triboelectric effect takes place at the contact between the grains and at the contact between the grains and the device. Therefore, the characteristics of the powder and the nature of the material used to build the device are important parameters.
LEARN MORE ABOUT THE GRANUCHARGE
GranuCharge instrument measures automatically and precisely the quantity of electrostatic charges created inside a powder during a flow in contact with a selected material.
The powder sample flows inside a vibrating V-tube and fall in a Faraday cup connected to an electrometer. The electrometer measures the charge acquired by the powder during the flow inside the V-tube. In order to obtain reproducible results, a rotating or a vibrating device is used to feed the V-tube regularly.
Powder description
One sample has been selected for this application note. It is a glass powder which has been coated with a copolymer of ethylene grafted with succinic anhydride (ESA). This copolymer contains succinic anhydride rings (Figure 1a) that hydrolyses into succinic acid upon exposure to moisture (Figure 1b). As succinic acid is a weak acid, it also partially dissociates in water (Figure 1c).
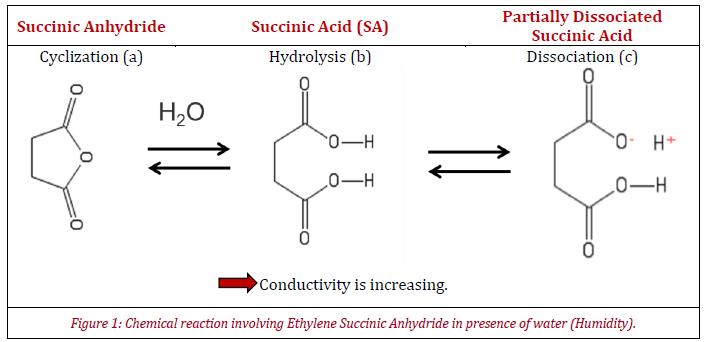
Figure 1 shows that at low humidity, anhydride rings are closed and show low conductivity, while the hydrolyzed succinic acid exhibits higher conductivity upon dissociation in presence of water. Thus, a high conductivity means a good ability to dissipate electrical charges, while a sample with low conductivity will have difficulty to dissipate electrical charges.
GranuCharge analysis
Experimental protocol
The triboelectric effect of the powders was investigated with the help of GranuCharge instrument. For each experiment with the GranuCharge, Stainless-steel 316L pipes and rotating feeder were used (cf. Figure 2):
The quantity of product used for each measure was 50g and the powder was not recycled after a measurement. Some tests have been repeated many times in order to show GranuCharge accuracy/reproducibility.
LEARN MORE ABOUT THE GRANUCHARGE
Before experiment, powder mass (mp, in g) is recorded. At the beginning of the test, the initial powder charge (Qi, in μC) is measured by introducing powder inside the Faraday cup. Once these steps are completed, the powder is poured inside the rotating feeder, then experiment is started. Final charge is measured at the end of experiment (Qf, μC).
Two different tests were carried out, the first one investigates the influence of a low moisture content (at RH = 38% and T = 25°C) and the second one shows the influence of a high moisture content on the charge density (at RH = 85% and T = 80°C).
For each test, the measurement protocol is the same. The first point (at t = 0 min) corresponds to ambient air conditions (after opening the sample box). The second point concerns sample electrical charge after one hour in an oven at 170°C. All last points are recorded after putting the samples in contact with humidity.
Results
The next tables summarize the raw results obtained for the first test and the second one:
| Sample in ambiant air (RH=38%, T=25°C) | |
|---|---|
| Recovery Time (min) | Charge Density q (µC/kg) |
| 0 | 0.000 |
| 0 | 0.239 |
| 35 | 0.250 |
| 60 | 0.217 |
| 87 | 0.222 |
| 153 | 0.225 |
| 1043 | 0.194 |
| Sample in contact with water bath at 80°C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Recovery Time (min) | mp (g) | Test n° | Initial Charge Qi (µC) | Final Charge Qf (µC) | Qf - Qi (µC) | Charge Density q (µC/kg) | Mean q (µC/kg) | Standard Deviation | Comments |
| 0 | 50 | 1 | 0.0107 | 0.018 | 0.0073 | 0.1460 | 0.1982 | 0.0318 | Reference Sample |
| 0 | 50 | 2 | 0.00836 | 0.0173 | 0.00894 | 0.1788 | |||
| 0 | 50 | 3 | 0.00711 | 0.0188 | 0.001169 | 0.2338 | |||
| 0 | 50 | 4 | 0.00792 | 0.0187 | 0.01078 | 0.2156 | |||
| 0 | 50 | 5 | 0.0043 | 0.0152 | 0.0109 | 0.2180 | |||
| 0 | 50 | 6 | 0.00674 | 0.0166 | 0.00986 | 0.1972 | |||
| 0 | 50 | 1 | 0.00382 | 0.026 | 0.02218 | 0.4436 | 0.4528 | 0.0130 | 1h at 170°C |
| 0 | 50 | 2 | 0.0186 | 0.0417 | 0.0231 | 0.4620 | |||
| 35 | 50 | 1 | -0.002 | 0.0107 | 0.0127 | 0.2540 | 0.2540 | 0.0000 | After 35 min |
| 150 | 50 | 1 | -0.0272 | -0.0164 | 0.0108 | 0.2160 | 0.2160 | 0.0000 | After 150 min |
| 1140 | 50 | 1 | -0.0239 | -0.0255 | -0.0016 | -0.0320 | -0.0320 | 0.0000 | After 1140 min |
The following figure represents charge density variation (Mean q, in μC/kg) versus time of experiment:
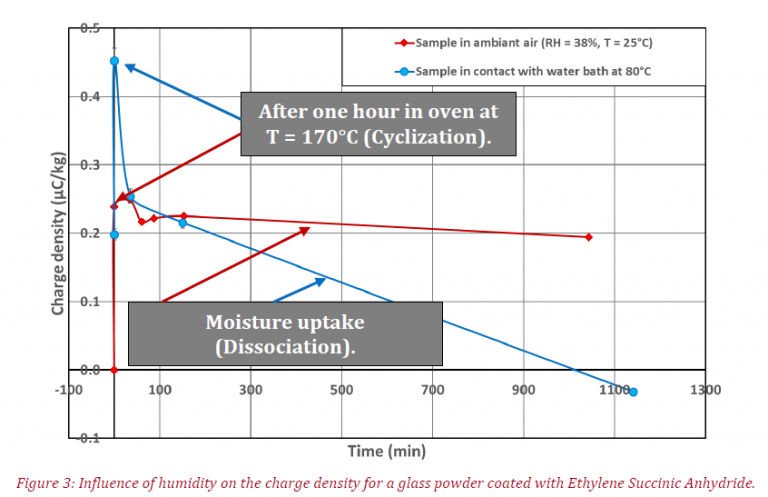
These results are highly interesting, at low moisture content (red curve) a small decrease of electrical charge is highlighted after 1140min in contact with humidity. The modifications of the coating properties are the cause (cf. Figure 1), this is due to the transition between structure (a) to (c).
However, at high humidity (blue curve) the transition between those two organic compounds is faster, this is the same trend for charge density variation (because compound (c) is anti-static due to its high conductivity).
The difference between the first points in charge density for the two tests can be explained by the fact that the chemical reaction is a chemical equilibrium (cf. Figure 1). Thus, after taking the sample out of its cardboard box, it is possible that the coating composition of the glass powder corresponds to a blend of forms (a), (b) and (c).
At the end of experiment for test with high moisture value (blue curve), the sample has a negative charge density, this fact may be of the coating (ESA) which is originally slightly negatively charged.
LEARN MORE ABOUT THE GRANUCHARGE
Conclusions
- The GranuCharge instrument can show some modifications in the surface properties of a powder;
- The GranuCharge measures the influence of humidity on the coating of a glass.
Bibliography
Cascade of granular flows for characterizing segregation, G. Lumay, F. Boschin, R. Cloots, N. Vandewalle, Powder Technology 234, 32-36 (2013).
Combined effect of moisture and electrostatic charges on powder flow, A. Rescaglio, J. Schockmel, N. Vandewalle and G. Lumay, EPJ Web of Conferences 140, 13009 (2017).
Compaction dynamics of a magnetized powder, G. Lumay, S. Dorbolo and N. Vandewalle, Physical Review E 80, 041302 (2009).
Compaction of anisotropic granular materials: Experiments and simulations, G. Lumay and N. Vandewalle, Physical Review E 70, 051314 (2004).
Compaction Dynamics ofWet Granular Assemblies, J. E. Fiscina, G. Lumay, F. Ludewig and N. Vandewalle, Physical Review Letters 105, 048001 (2010).
Effect of an electric field on an intermittent granular flow, E. Mersch, G. Lumay, F. Boschini, and N. Vandewalle, Physical Review E 81, 041309 (2010).
Effect of relative air humidity on the flowability of lactose powders, G. Lumay, K. Traina, F. Boschini, V. Delaval, A. Rescaglio, R. Cloots and N. Vandewalle, Journal of Drug Delivery Science and Technology 35, 207-212 (2016).
Experimental Study of Granular Compaction Dynamics at Different Scales: Grain Mobility, Hexagonal Domains, and Packing Fraction, G. Lumay and N. Vandewalle, Physical Review Letters 95, 028002 (2005).
Flow abilities of powders and granular materials evidenced from dynamical tap density measurement, K. Traina, R. Cloots, S. Bontempi, G. Lumay, N. Vandewalle and F. Boschini, Powder Technology, 235, 842-852 (2013).
Flow of magnetized grains in a rotating drum, G. Lumay and N. Vandewalle, Physical Review E 82, 040301(R) (2010).
How tribo-electric charges modify powder flowability, A. Rescaglio, J. Schockmel, F. Francqui, N. Vandewalle, and G. Lumay, Annual Transactions of The Nordic Rheology Society 25, 17-21 (2016).
Influence of cohesives forces on the macroscopic properties of granular assemblies, G. Lumay, J. Fiscina, F. Ludewig and N. Vandewalle, AIP Conference Proceedings 1542, 995 (2013).
Linking compaction dynamics to the flow properties of powders, G. Lumay, N. Vandewalle, C. Bodson, L. Delattre and O. Gerasimov, Applied Physics Letters 89, 093505 (2006).
Linking flowability and granulometry of lactose powders, F. Boschini, V. Delaval, K. Traina, N. Vandewalle, and G. Lumay, International Journal of Pharmaceutics 494, 312–320 (2015).
Measuring the flowing properties of powders and grains, G. Lumay, F. Boschini, K. Traina, S. Bontempi, J.-C. Remy, R. Cloots, and N. Vandewall, Powder Technology 224, 19-27 (2012).
Motion of carbon nanotubes in a rotating drum: The dynamic angle of repose and a bed behavior diagram, S. L. Pirard, G. Lumay, N. Vandewalle, J-P. Pirard, Chemical Engineering Journal 146, 143-147 (2009).
Mullite coatings on ceramic substrates: Stabilisation of Al2O3–SiO2 suspensions for spray drying of composite granules suitable for reactive plasma spraying, A. Schrijnemakers, S. André, G. Lumay, N. Vandewalle, F. Boschini, R. Cloots and B. Vertruyen, Journal of the European Ceramic Society 29, 2169–2175 (2009).
Rheological behavior of β-Ti and NiTi powders produced by atomization for SLM production of open porous orthopedic implants, G. Yablokova, M. Speirs, J. Van Humbeeck, J.-P. Kruth, J. Schrooten, R. Cloots, F. Boschini, G. Lumay, J. Luyten, Powder Technology 283, 199–209 (2015).
The influence of grain shape, friction and cohesion on granular compaction dynamics, N. Vandewalle, G. Lumay, O. Gerasimov and F. Ludewig, The European Physical Journal E (2007).

