Pharmaceutical
Rheological Study of Blends for Dry Powder Inhalers (DPI) Applications (GranuPack and GranuDrum analyses)
One of the biggest advantages of the Dry Powder Inhaler (DPI) is that it allows a better preservation of the formulation due to the absence of water in the formulation...
Introduction

The divided solid materials have a considerable share in the products manufactured or used in the pharmaceutical industries (excipients, active pharmaceutical ingredient – API, additives, etc).
One of the big challenges of this century is to characterize their various physical behaviours. Nowadays, the stress is mainly putted on the study of the relations relating the grain properties to these of the powders bed. The purpose is thus to be able to control, analyze and improve the manufacturing processes of the powders.
One of the biggest advantages of the Dry Powder Inhaler (DPI) is that it allows a better preservation of the formulation due to the absence of water in the formulation. These inhalers are commonly used to treat the respiratory diseases like asthma, bronchitis, emphysema and the obstructive chronic broncho-pneumopathy. They also allow to treating the sweetened diabetes.
Most of the capsules used in these inhalers contain micronized active drug (API) mixed with coarse particles (carriers or excipients). The carriers prevent the segregation in the blend and give a better flow ability to the formulation.
The generation of the aerosol is produced by the actuation of the particles from introduced air through various mechanisms of shearing and/or turbulence. The powder bed enters then in the lungs of the patient where the drug particles are separated from the carrier before to reach the major parts of the lungs.
For most DPI applications, the main challenge consists in optimizing the formulation in order to obtain the largest FPF (fine particle fraction) during the inhalation. For this, to control the flow ability of the powder is a key parameter. Indeed, good flow properties allow, on the one hand, keeping the capsules filling regular and, on the other hand, ensure a reproductible aerosol production which guarantees the amount of drug delivery to the patient. Blends are thus characterized in order to measure flow ability, ventilation, cohesion and compressibility of some new developed DPI formulations.
Materials And Methods
Four formulations containing each one a similar excipient (Lactose: L) and two active drugs (A, B or C) were prepared in different ratios (ABL-20-2-5, ABL-20-2-10, ACL-20-2-20 and ABL-20-2-30). These blends are then characterized by using new instruments (GranuPack, GranuDrum and GranuHeap), scanning electron microscopy and particle size analyzer.
In this application note, correlations between rheological properties (GranuDrum), packing measurement (GranuPack) and grain size distributions are done. Flow robustness powder is discussed.
LEARN MORE ABOUT THE GRANUPACK
| Sample Name | API | Lactose | |
|---|---|---|---|
| A | B or C | L | |
| ABL 20-2-5 | 20 | 2 | 5 |
| ABL 20-2-10 | 20 | 2 | 10 |
| ABL 20-2-30 | 20 | 2 | 30 |
| ACL 20-20 | 20 | 2 | 20 |
Table 1: Pharmaceutical formulations prepared containing a similar excipient (Lactose: L) and two active drugs (A, B or C)
Results and Discussions
By comparison with the excipient grain size distribution (lactose – yellow discs on the figure 1), the analysis of the grain size distributions of the blends shows that the last mode is associated with the lactose grain particles.
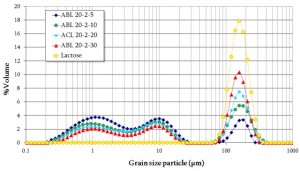
The first two modes belong to the API’s subpopulations.
Note that the increase of the area under the last mode is not astonishing; the excipient volume fraction being reinforced as follows: ABL 20-2-5 < ABL 20-2-10 < ACL 20-2-20 < ABL 20-2-30.
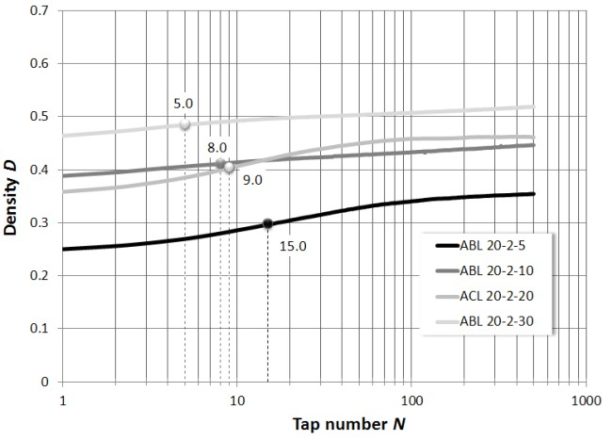
Figure 2: Dynamical tap test measurement for pharmaceutical blends – GranuPack
The ABL-20-2-5 blend shows the largest variation. Its initial density is the lowest and the packing/compressing kinetic (N1/2 = 15.0) is the slowest (see the application notes Dynamic Tap Density Tester as a useful tool for analyzing pharmaceutical powders for explanations of N1/2).
Contrary to the curves of the samples ABL-20-2-30 and ABL-20-2-10 which have a linear appearance (non-cohesive behaviour), the curves of the samples ABL-20-2-5 and ACL-20-2-20 are sigmoid (cohesive behaviour) specifying a particulate reorganization preliminary to compressing.
The analyses of the rheological dry powder measurement results obtained from GranuDrum (see cohesive indexes curves on figure 3) show that ABL-20-2-10 and ABL-20-2-30 blends are more robust than ABL-20-2-5 and ACL-20-2-20. Cohesive indexes measurements highlight a disparity in the behavior of the go and back curves for the ABL-20-2-5 and ACL-20-2-20 blends. Indeed, at lowest speeds, the back-flow angles are weaker than the go one’s.
LEARN MORE ABOUT THE GRANUDRUM
These observations are to be related to the presence of caked balls shaped during the measurement process. These balls limit the friction and agglomerates contact number and reduce the cohesion of the granular system.
The hysteresis quantification of the cohesive indexes’ measurement gives information on the ability of the blends to preserve its flow properties in a reversible way.
The classification of the powder flow robustness is obtained from the Table 2 (best results on the right): ABL-20-2-5 < ACL-20-2-20 < ABL-20-2-10 < ABL-20-2-30.
.jpeg)
Figure 3: Flow robustness measurement of pharmaceutical blends for dry powder inhalers (DPI)
| Sample Name | Robustness (°.rpm) |
|---|---|
| ABL 20-2-5 | 172 |
| ABL 20-2-10 | 13 |
| ACL 20-2-20 | 108 |
| ABL 20-2-30 | 6 |
Table 2: Flow robustness measurement of pharmaceutical blends for dry powder inhalers
Conclusion
The measurements done on the formulations show that ABL-20-2-10 and ABL-20-2-30 blends show the best flow properties. The formulation ABL-20-2-5 shows the poorest performances for capsules filling. The ACL-20-2-20 formulation is between these two extremes.
The characterization of the rheological powder properties (flow, ventilation, compressing) offers multiple advantages. It ensures a better-quality control of the batches, and this, at the various stages of their production. It avoids spending money or useless losses of time: the optimization of the new formulations is done immediately during their development in laboratory. In the case of DPI applications, correlation between rheological properties and FPF can be done.
