Granular materials and fine powders are widely used in industrial applications. To control and to optimize processing methods, these materials have to be precisely characterized. The characterization methods are related either to the properties of the grains (granulometry, morphology, chemical composition, …) and to the behavior of the bulk powder (flowability, density, blend stability, electrostatic properties, …).
The chemistry of the particle or the coating can change the behavior of a powder. In that sense, Granutools made several studies on chemicals to understand the impact of such change on powder flowability.
One sample has been selected for this application note. It is a glass powder that has been coated with a copolymer of ethylene grafted with succinic anhydride (ESA). This copolymer contains succinic anhydride rings (Figure 1a) that hydrolyze into succinic acid upon exposure to moisture (Figure 1b). As succinic acid is a weak acid, it also partially dissociates in water (Figure 1c).
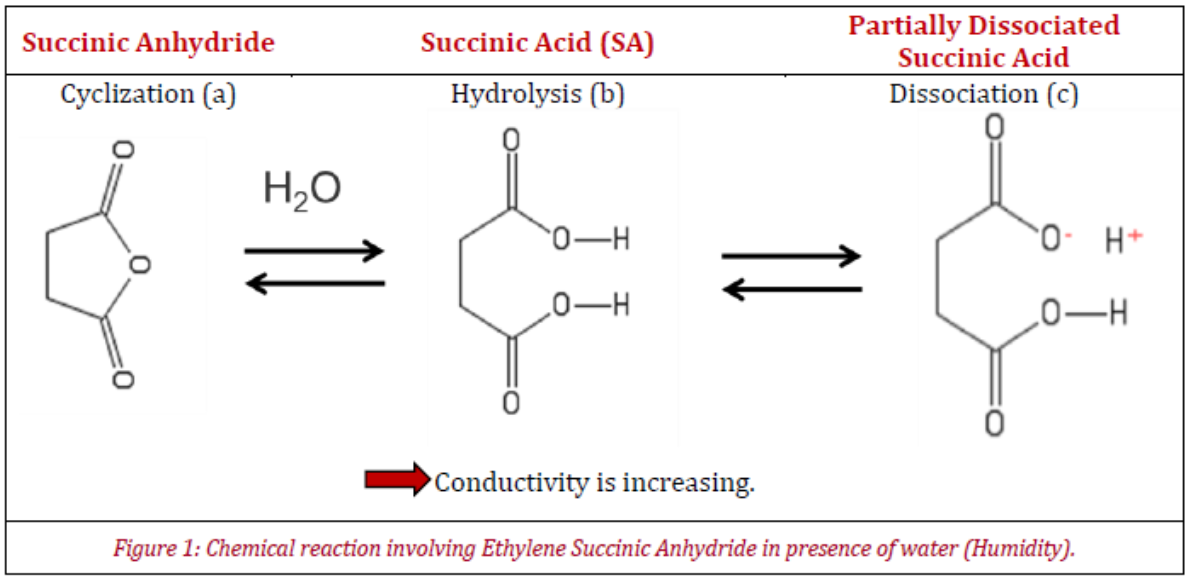 Figure 1 shows that at low humidity, anhydride rings are closed and show low conductivity, while the hydrolyzed succinic acid exhibits higher conductivity upon dissociation in presence of water. Thus, a high conductivity means a good ability to dissipate electrical charges, while a sample with low conductivity will have difficulty to dissipate electrical charges.
Figure 1 shows that at low humidity, anhydride rings are closed and show low conductivity, while the hydrolyzed succinic acid exhibits higher conductivity upon dissociation in presence of water. Thus, a high conductivity means a good ability to dissipate electrical charges, while a sample with low conductivity will have difficulty to dissipate electrical charges.
Reference: Granutools Application Notes (2016)
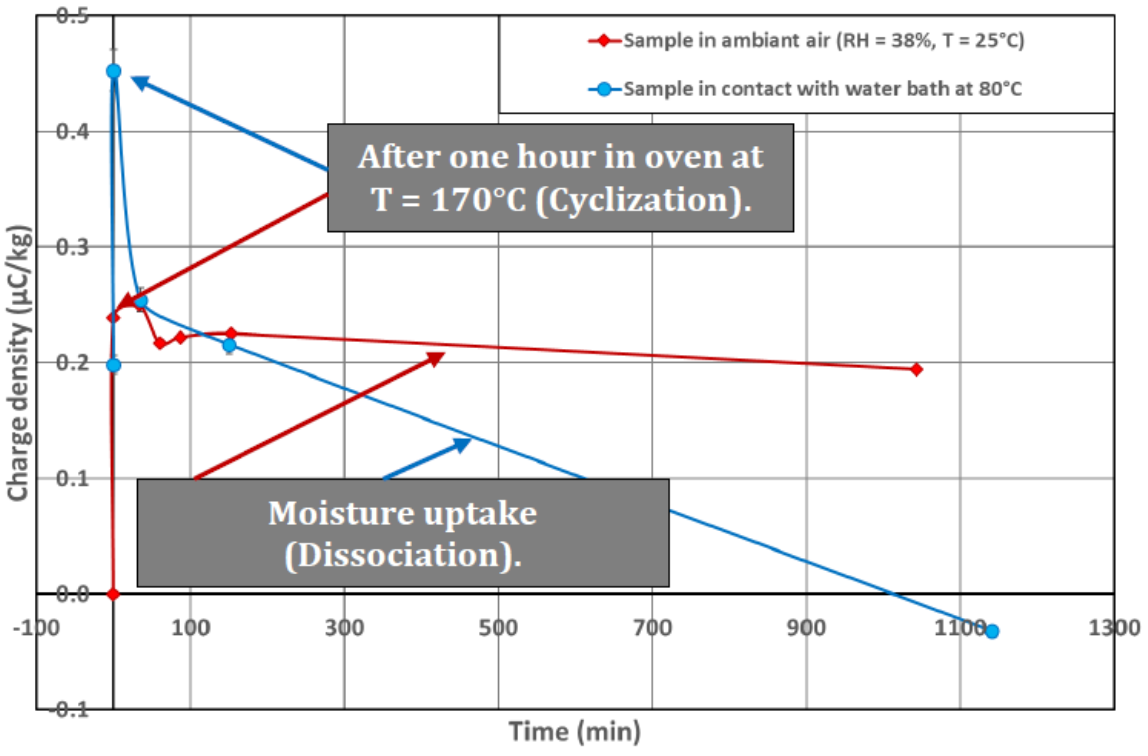
These results are highly interesting, at the low moisture content (red curve) a small decrease of electrical charge is highlighted after 1140min in contact with humidity. The modifications of the coating properties are the cause (cf. Figure 1), this is due to the transition between structure (a) to (c).
However, at high humidity (blue curve) the transition between those two organic compounds is faster, this is the same trend for charge density variation (because compound (c) is anti-static due to its high conductivity).
The difference between the first points in charge density for the two tests can be explained by the fact that the chemical reaction is a chemical equilibrium (cf. Figure 1). Thus, after taking the sample out of its cardboard box, it is possible that the coating composition of the glass powder corresponds to a blend of forms (a), (b), and (c).
At the end of the experiment for the test with high moisture value (blue curve), the sample has a negative charge density, this fact may be of the coating (ESA) which is originally slightly negatively charged.