Powders are extensively used in pharmaceutical applications as excipients or active ingredients in formulations. Numerous production processes, such as tableting, wet and dry granulation, blending, and capsule filling, involve the handling of powders. Therefore, advancements in understanding powder flow behavior significantly impact pharmaceutical manufacturing. Poor powder flow properties lead to serious production issues like clogging, agglomeration, and segregation.
Our Standardization and Automation Workflow simplifies powder characterization with GranuFlow, GranuHeap, and GranuPack, enabling standardized, automated measurements for greater efficiency and consistency. For pharmaceutical companies, compliance with Pharmacopeia quality standards is crucial. While strictly adhering to these standards, our instruments go beyond basic compliance to provide deeper insights and enhanced process control. GranuPack ensures standardized tapped density measurements (USP 616), GranuFlow is the only automatic funnel flowmeter meeting industry standards, and GranuHeap performs USP 1174 Angle of Repose measurements with full automation, minimizing operator dependency.
We also offer GranuDrum to optimize tableting, powder flow, and blend uniformity, and GranuCharge to improve continuous manufacturing, electrostatic control, and powder handling.
All our instruments align with Process Analytical Technology (PAT) and Quality by Design (QbD) principles, ensuring high-quality products through real-time monitoring. They comply with Good Manufacturing Practices (GMP) and are 21 CFR Part 11 compliant, meeting FDA requirements for electronic records and signatures.
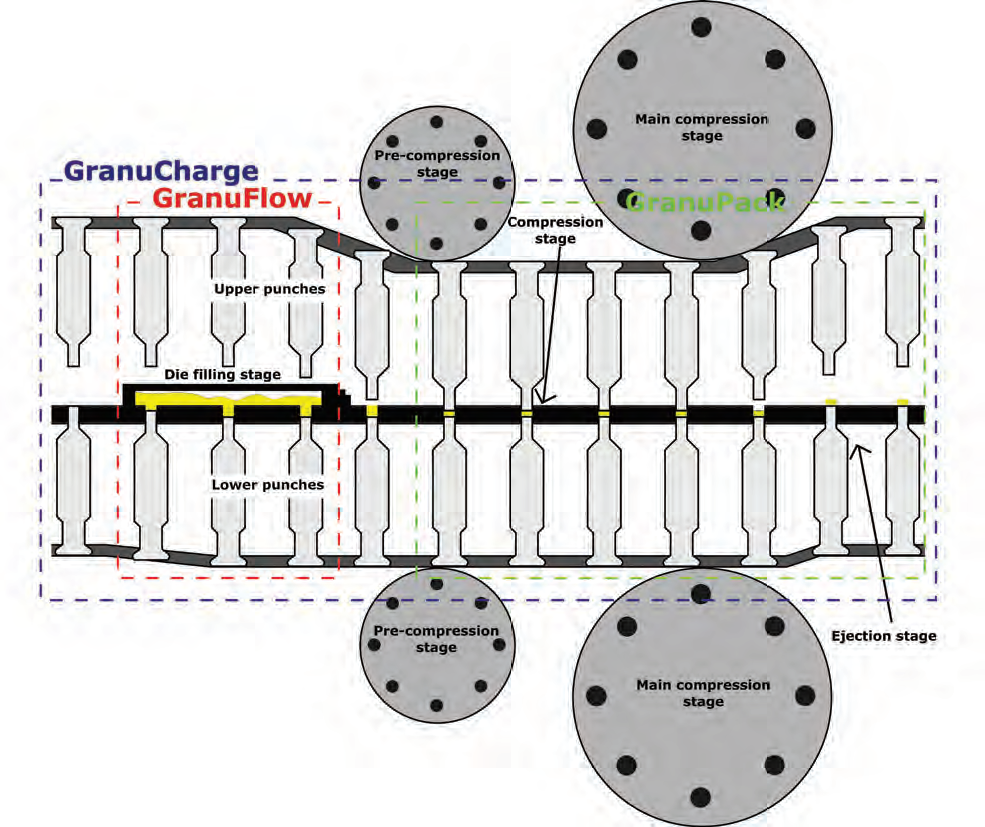
TO OPTIMIZE TABLETING PROCESS, GRANUTOOLS OFFERS A WORKFLOW OF TWO INSTRUMENTS: GRANUFLOW FOR DIE FILLING, AND GRANUPACK FOR POWDERS PACKING.
In pharmaceutical industries, it is well-known that the variation in composition and the quality of tablets are determined by material properties and process conditions.
Tablets are manufactured by compressing dry powders or granules in a die. This process consists of three primary stages: die filling, compaction, and ejection. Powders flowability and compressibility during the die filling process controls the tablet composition, the tablet properties as well as homogeneity. Therefore, the study of die filling process parameters has a significant role in controlling the tablet manufacturing industry.
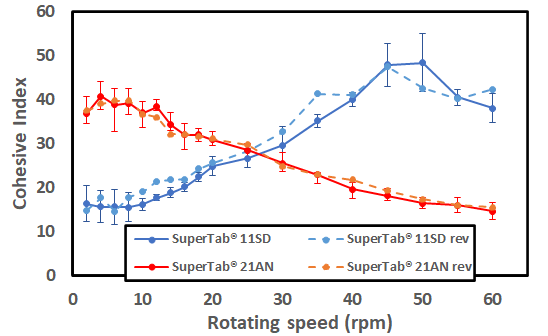
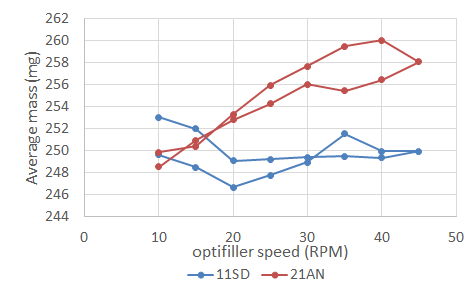
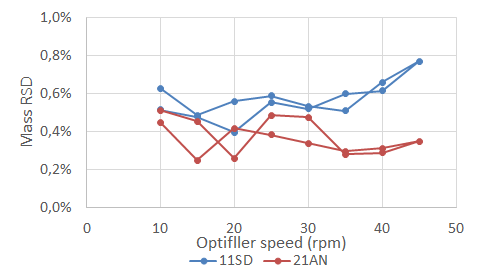
A more recent publication explains the dynamic performance of various excipients through the optifiller of a tableting machine using the GranuDrum. In collaboration with DFE Pharma, Granutools published some results showing the link between the cohesive index of the Super Tab and the mass RSD of the tablets in a rotary press. In this case, we can see below how the behavior at low and high speed will impact the average mass and RSD of the final product.
Reference: On Drug Delivery (2020), Neveu and al.
Inhalers have been developed because of difficulties when using the conventional metered-dose inhaler (MDI). Dry powder inhalers (DPI) in general are easier to use than the MDI and cause fewer irritant effects. Unlike the MDI few patients develop a poor inhalation technique with continued use of DPI.
The inspiratory flow necessary to achieve a therapeutic effect is critical with Dry Powder Inhalers (DPI). Moreover, this technology faces various problems: powder aging, agglomeration induced by electrostatic charges, blend homogeneity during capsule filling, …
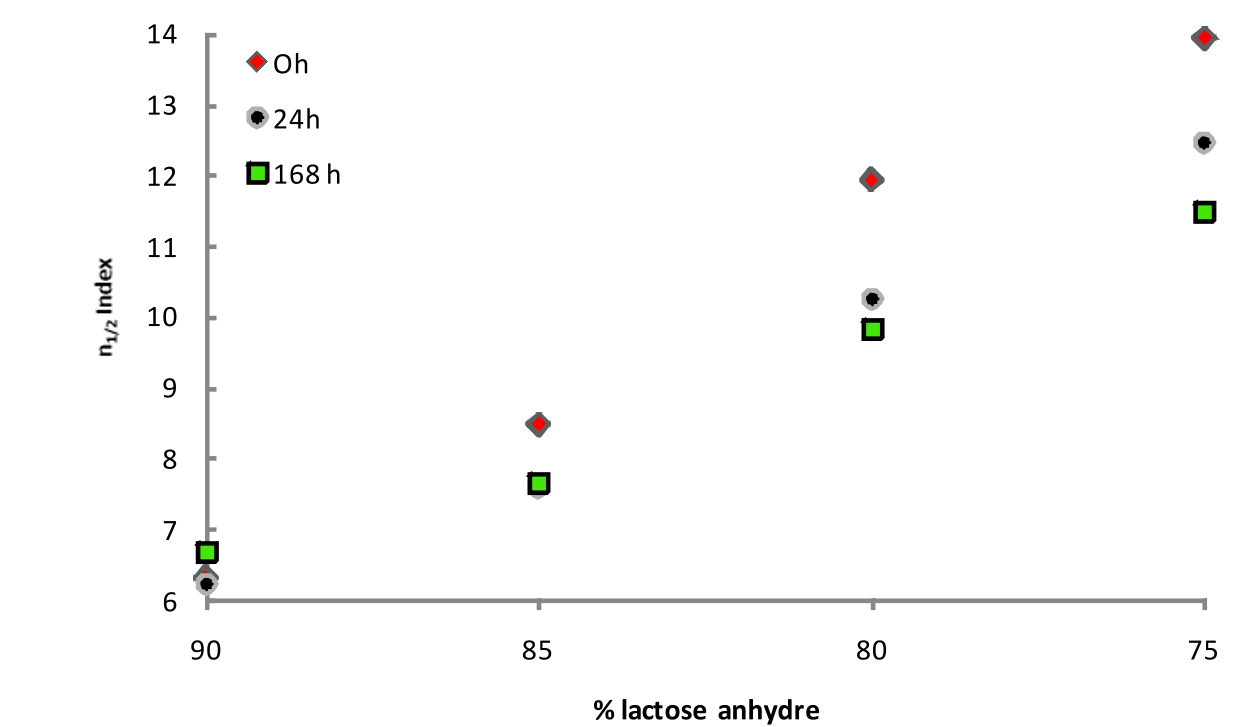
Here is an example of the aging effect taking place in a DPI formulation, reference Granutools, Application notes, 2016.
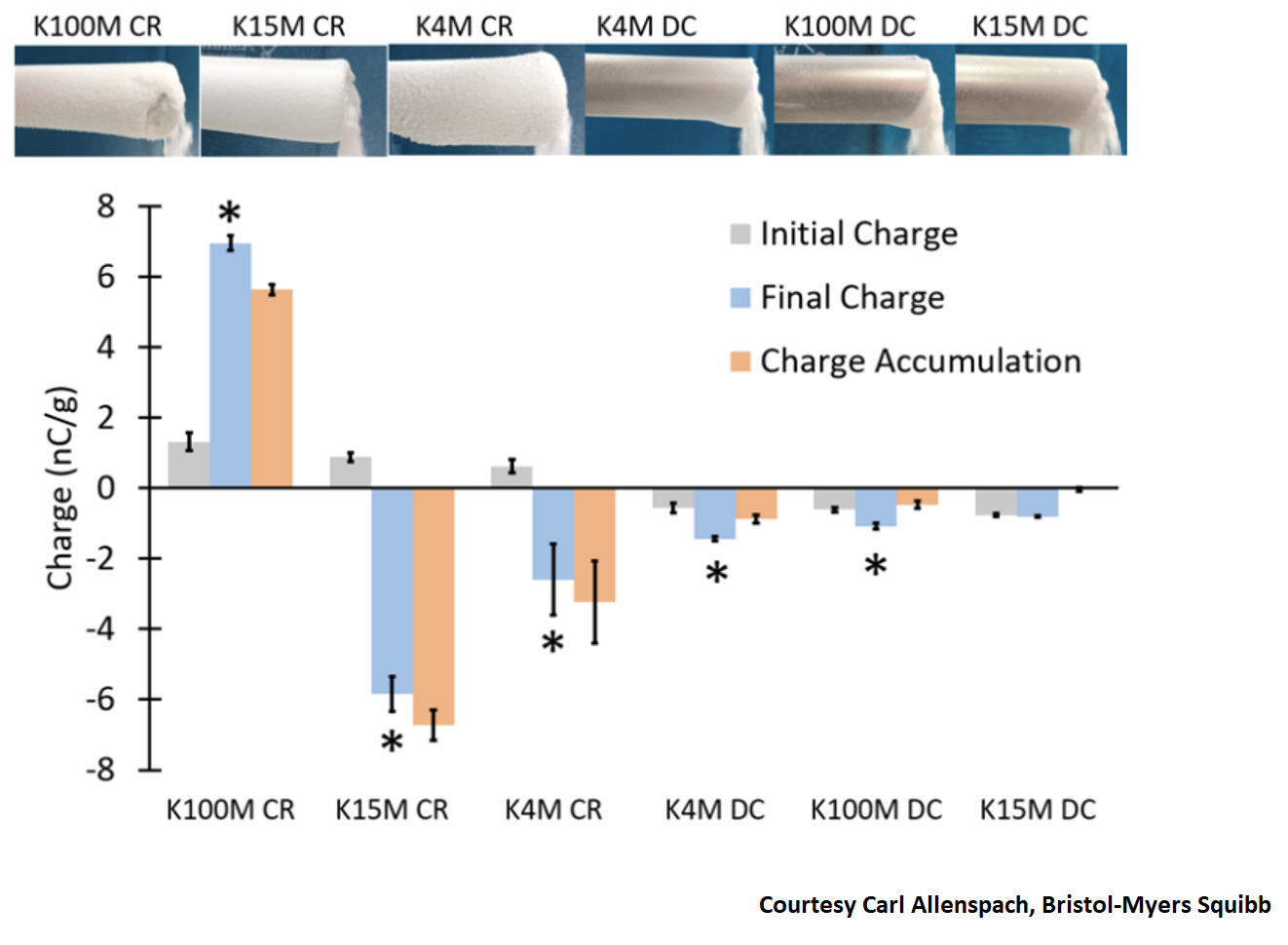
The presence of electrostatic charges inside a powder is known to influence drastically the material flow properties. The triboelectric charges produced at the contacts between the grains and the contacts between the grains and the container produces electrostatic forces. On the one hand, the triboelectric effect is useful for many applications, but on the other hand, the triboelectrification causes complications (powder sticking on pipes surface, agglomeration). Thus, to help industries to quantify this phenomenon GranuTools develops the GranuCharge instrument. It measures automatically and precisely the amount of electrostatic charges created inside a powder during a flow in contact with a selected material.
Electrostatics is becoming more challenging moving from batch to continuous manufacturing due to the charge build-up inside the process line.
Here is an example of agglomeration occurring in the dispensing unit of a continuous manufacturing process and the correlated measurement using the Granucharge